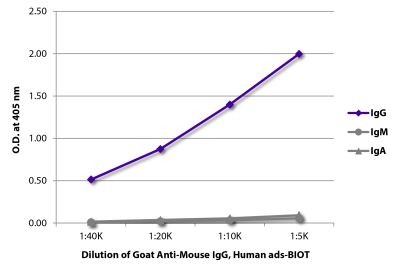
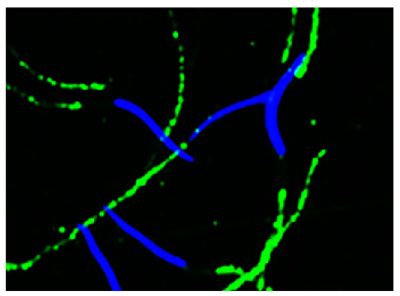
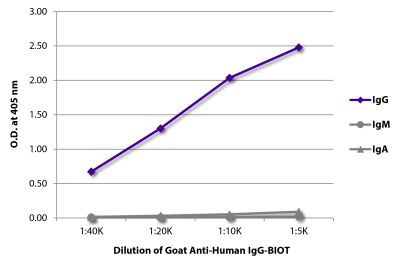
Streptavidin-APC
Only %1 left
Cat. No.:
7105-11L,
7105-11M,
7105-11S
Streptavidin-APC for use in flow cytometry assays.
As low as
$116.00


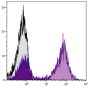
| Specificity | Biotin |
|---|---|
| Source | E. coli |
| Conjugate | APC (Allophycocyanin) |
| Buffer Formulation | Phosphate buffered saline containing < 0.1% sodium azide and a stabilizer |
| Concentration | 0.5 mg/mL |
| Volume | 0.2 mL, 1.0 mL, or 2.0 mL |
| Recommended Storage | 2-8°C; Avoid exposure to light; Do not freeze |
| Applications |
Quality tested applications for relevant formats include - ELISA 1-11 FLISA Flow Cytometry 12,13 Other referenced applications for relevant formats include - ELISpot 14-17 Immunohistochemistry-Frozen Sections 21 Immunohistochemistry-Paraffin Sections 18 Immunocytochemistry 21 Western Blot 8,11 Multiplex 19,20 |
Documentation
Certificate of Analysis Lookup
Enter the Catalog Number and Lot Number for the Certificate of Analysis you wish to view
- 1. Pohlmeier L, Sonar SS, Rodewald H, Kopf M, Tortola L. Comparative analysis of the role of mast cells in murine asthma models using Kit-sufficient mast cell-deficient animals. Allergy. 2021;76:2030-43. (ELISA)
- 2. Honjo K, Russell RM, Li R, Liu W, Stoltz R, Tabengwa EM, et al. Convalescent plasma-mediated resolution of COVID-19 in a patient with humoral immunodeficiency. Cell Rep Med. 2020;2:100164. (ELISA)
- 3. Hayuningtyas RA, Han M, Choi S, Kwak MS, Park IH, Lee J, et al. The collagen structure of C1q induces wound healing by engaging discoidin domain receptor 2. Mol Med. 2021;27:125. (ELISA)
- 4. Afkhami S, D'Agostino MR, Zhang A, Stacey HD, Marzok A, Kang A, et al. Respiratory mucosal delivery of next-generation COVID-19 vaccine provides robust protection against both ancestral and variant strains of SARS-CoV-2. Cell. 2022;185:896-915. (ELISA)
- 5. Myzithras M, Lin S, Radden L, Hess Kenny C, Cai Z, MacDonald A, et al. Development of novel ultra-sensitive IL-11 target engagement assays to support mechanistic PK/PD modeling for an anti-IL-11 antibody therapeutic. Mabs. 2022;14:2104153. (ELISA)
- 6. Ekanger CT, Zhou F, Bohan D, Lotsberg ML, Ramnefjell M, Hoareau L, et al. Human organotypic airway and lung organoid cells of bronchiolar and alveolar differentiation are permissive to infection by influenza and SARS-CoV-2 respiratory virus. Front Cell Infect Microbiol. 2022;12:841447. (ELISA)
- 7. Ratswohl C, Vázquez García C, Ahmad AUW, Gonschior H, Lebedin M, Silvis CE, et al. A design strategy to generate a SARS-CoV-2 RBD vaccine that abrogates ACE2 binding and improves neutralizing antibody responses. Eur J Immunol. 2023;53:e2350408. (ELISA)
- 8. Tjärnhage E, Brown D, Bogen B, Andersen TK, Grødeland G. Trimeric, APC-targeted subunit vaccines protect mice against seasonal and pandemic influenza. J Virol. 2023;97:e0169422. (ELISA, WB)9.
- Dangi T, Sanchez S, Lew MH, Awakoaiye B, Visvabharathy L, Richner JM. Pre-existing immunity modulates responses to mRNA boosters. Cell Rep. 2023;42:112167. (ELISA)
- 10. Remy C, Pintado E, Dunlop M, Schön S, Kleinpeter P, Rozanes H, et al. Design and selection of anti-PD-L1 single-domain antibody and tumor necrosis factor superfamily ligands for an optimal vectorization in an oncolytic virus. Front Bioeng Biotechnol. 2023;11:1247802. (ELISA)
- 11. Wei Y, Chen Q, Chen J, Zhou C, Geng S, Shi D, et al. Loss of α-1,2-mannosidase MAN1C1 promotes tumorigenesis of intrahepatic cholangiocarcinoma through enhancing CD133-FIP200 interaction. Cell Rep. 2023;42:113588. (ELISA, WB)
- 12. Liu H, Zhao Q, Tan L, Wu X, Huang R, Zuo Y, et al. Neutralizing IL-8 potentiates immune checkpoint blockade efficacy for glioma. Cancer Cell. 2023;41:693-710.e8. (FC)
- 13. Smith ES, Balch LA, Scrivens M, Shi S, Wang W, Harvey CD, et al. Use of poxvirus display to select antibodies specific for complex membrane antigens. Mabs. 2023;15:2249947. (FC)
- 14. Dagotto G, Ventura JD, Martinez DR, Anioke T, Chung BS, Siamatu M, et al. Immunogenicity and protective efficacy of a rhesus adenoviral vaccine targeting conserved COVID-19 replication transcription complex. NPJ Vaccines. 2022;7:125. (ELISpot)
- 15. Garcia-Dominguez D, Henry C, Ma L, Jani H, Amato NJ, Manning T, et al. Altering the mRNA-1273 dosing interval impacts the kinetics, quality, and magnitude of immune responses in mice. Front Immunol. 2022;13:948335. (ELISpot)
- 16. Fan J, Li S, Zhang Y, Zheng J, Wang D, Liao Y, et al. Early emerging SARS-CoV-2 spike mutants are diversified in virologic properties but elicit compromised antibody responses. Viruses. 2023;15:2401. (ELISpot)
- 17. Huang H, Li Y, Zhang G, Ruan G, Zhu Z, Chen W, et al. The RNA-binding protein hnRNP F is required for the germinal center B cell response. Nat Commun. 2023;14:1731. (ELISpot)
- 18. Horii Y, Iniwa T, Onitsuka M, Tsukimoto J, Tanaka Y, Ike H, et al. Reversal of neuroinflammation in novel GS model mice by single i.c.v. administration of CHO-derived rhCTSA precursor protein. Mol Ther Methods Clin Dev. 2022;25:297-309. (IHC-PS)
- 19. Kalimuddin S, Tham CY, Qui M, de Alwis R, Sim JX, Lim JM, et al. Early T cell and binding antibody responses are associated with COVID-19 RNA vaccine efficacy onset. Med. 2021;2:682-88. (Multiplex)
- 20. Aitken EH, Damelang T, Ortega-Pajares A, Alemu A, Hasang W, Dini S, et al. Developing a multivariate prediction model of antibody features associated with protection of malaria-infected pregnant women from placental malaria. Elife. 2021;10:e65776. (Multiplex)
- 21. SouthernBiotech unpublished data (IHC-FS, ICC)
See All References