Share a link
Introduction to Immunocytochemistry Assays
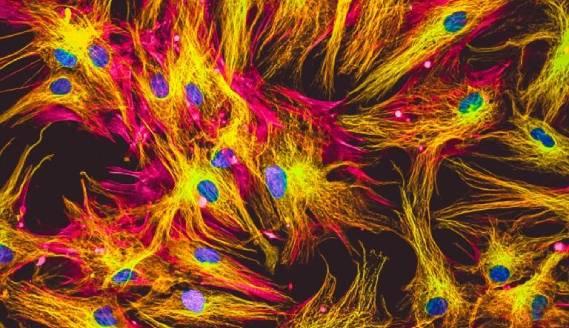
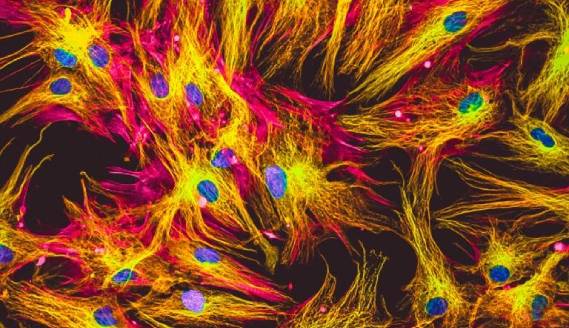
Immunocytochemistry (ICC) is a technique which uses antibodies for visualizing analytes of interest on, or within, cells. It is distinct from immunohistochemistry (IHC) and immunofluorescence (IF), yet the three terms are sometimes mistakenly interchanged, leading to confusion during antibody selection.
How is ICC different from IHC and IF?
ICC describes the process of immunostaining intact cells. Sample types that are studied with ICC include adherent cells which have been cultured as a monolayer in optical-bottomed microplates or on glass cover slips, and suspension cells which have been deposited onto glass slides via centrifugation (e.g., the Cytospin™ method) or in the form of a smear (e.g., blood and swab samples). In addition, ICC is applicable to the study of 3-dimensional cell cultures such as organoids or spheroids, although these types of experiments may have some subtle protocol differences. In contrast, IHC describes the process of immunostaining tissue samples, which may be either frozen or formalin fixed and paraffin-embedded (FFPE). IF is a form of detection, applicable to both ICC and IHC, whereby fluorophore-labeled antibodies are used for visualizing targets of interest.
What are the main steps in a typical ICC workflow?
A typical ICC protocol begins with fixation, which serves to preserve the cells at a set point in time and protect them during downstream processing. Fixation is usually achieved by incubating the cells in a solution comprising 1-4% paraformaldehyde (PFA) in phosphate buffered saline (PBS) for 10 minutes at room temperature, or in 100% methanol for 10 minutes at -20oC.
When staining for intracellular targets, PFA-fixed samples require permeabilization using a detergent such as Triton X-100, Tween-20, or NP-40, or an agent such as saponin, diluted to 0.1-0.5% v/v in PBS. However, permeabilization is not required when using methanol-fixed samples.
After fixation and/or permeabilization, and a series of PBS washes, the samples are blocked, usually with bovine serum albumin (BSA) or normal serum. The cells are then immunostained using either labeled primary antibodies, or a combination of unlabeled primary antibodies and labeled secondary antibodies with a wash step in between incubations.
We offer over 1,000 antibodies that have been validated for ICC as well as normal goat, rabbit, and donkey serum for blocking.
After further washing to remove any unbound antibody reagents, and counterstaining to detect nuclei (e.g., using DAPI or Hoechst), the cells are imaged using a microscope. This requires that cells in microplates be submerged in PBS, while cells on coverslips or glass slides must instead be mounted using an aqueous or solvent-based mounting media.
Our mounting media include Fluoromount-G®, which has more than 20,000 literature citations, and DAPI Fluoromount-G®, which includes DAPI for nuclear analysis.
Considerations for ICC antibody staining
Like any other immunostaining technique, ICC protocols must be tailored to the model system being studied. Each protocol step requires careful optimization, meaning everything from how the samples will be prepared through to factors such as reagent selection and incubation conditions (e.g., time, temperature, reagent concentrations).
When it comes to choosing antibody reagents for ICC, researchers face several decisions. These include whether to use labeled primary antibodies (direct detection) or whether to include a secondary antibody incubation step (indirect detection).
An advantage of using secondary antibodies is that they provide signal amplification, which increases assay sensitivity. Secondary antibodies are also often compatible with multiple applications, providing a degree of consistency across different workflows. However, if normal serum is to be used for blocking, it is important to ensure that secondary antibodies share the same host species to avoid unwanted cross-reactivities.
Our product portfolio includes over 1,400 labeled primary antibodies, as well as an extensive selection of secondary antibodies for species including mouse, human, rat, rabbit, and goat.
Another factor to consider is the type of readout that will be used. While ICC once relied on chromogenic detection, during which enzyme-labeled antibodies convert a chromogenic substrate to a colored precipitate at the target site, IF detection is now far more common. This is because IF detection allows for multiplexing, as well as removes the inherent user variability associated with monitoring and stopping chromogenic reactions.
We have developed a broad array of enzyme- and fluorophore-labeled antibodies, giving you maximum flexibility for your research.
If ICC will be multiplexed, it is critical to ensure that the antibodies being used are compatible. This means optimizing the immunostaining protocol for all of the antibodies in the panel - which could include staining for extracellular targets first, before fixing, permeabilizing and staining for intracellular targets, to avoid damaging extracellular epitopes - and confirming that antibodies do not cross-react. When several secondary antibodies are to be combined in the same experiment, using cross-adsorbed secondaries will minimize the risk of off-target binding.
Our HRP- and Alexa Fluor-conjugated secondary antibodies are cross-adsorbed against multiple species for results you can trust.