Hamster Anti-Mouse CD152-UNLB (1B8)
Cat. No.:
1790-01
Purified Anti-Mouse CD152 antibody for use in flow cytometry, ELISA, and stimulation assays.
$223.00
| Clone | 1B8 |
|---|---|
| Isotype | Hamster (Armenian) IgG1 |
| Isotype Control | Hamster IgG-UNLB |
| Specificity | Mouse CD152 |
| Alternative Names | CTLA-4, cytotoxic T-lymphocyte protein 4 |
| Description | The lymphocyte surface antigen CD152, also known as CTLA-4, is related to the costimulatory molecule CD28 and both molecules share common B7 family counter-receptors. However CD152 is thought to be a negative regulator of T cell activation and may play a role in apoptotic control of T cells. CD152 is relatively conserved among humans, mice, and chickens. |
| Immunogen | Extracellular portion of murine CTLA-4 fused to a murine IgG2a |
| Conjugate | UNLB (Unconjugated) |
| Buffer Formulation | Borate buffered saline, pH 8.2 |
| Clonality | Monoclonal |
| Concentration | 0.5 mg/mL |
| Volume | 1.0 mL |
| Recommended Storage | 2-8°C |
| Applications |
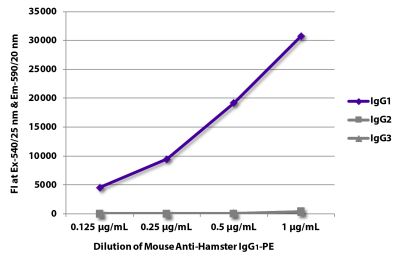
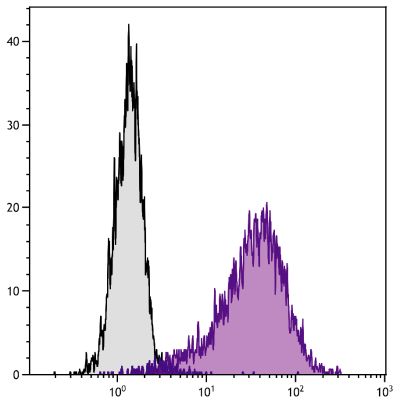
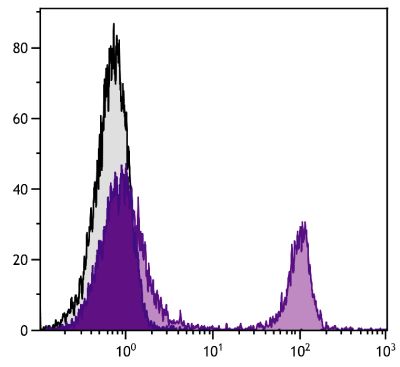
ELISA – Quality tested 2 FLISA – Quality tested Flow Cytometry – Reported literature 3-11 Stimulation – Reported in literature 1 |
| RRID Number | AB_2795291 |
| Gene ID |
12477 (Mouse) |
| Gene ID Symbol |
Ctla4 (Mouse) |
| Gene ID Aliases | Cd152; Ly-56; Ctla-4 |
| UniProt ID |
P09793 (Mouse |
| UniProt Name |
CTLA4_MOUSE (Mouse) |
Documentation
Certificate of Analysis Lookup
Enter the Catalog Number and Lot Number for the Certificate of Analysis you wish to view
- 1. Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405-13. (Immunogen, Stim)
- 2. Kimura F, Gotoh M, Tanaka T, Luo Z, Miyazaki J, Uede T, et al. Locally expressed CTLA4-Ig in a pancreatic beta-cell line suppresses accelerated graft rejection response induced by donor-specific transfusion. Diabetologia. 2002;45:831-40. (ELISA)
- 3. Ben-David H, Sela M, Mozes E. Down-regulation of myasthenogenic T cell responses by a dual altered peptide ligand via CD4+CD25+-regulated events leading to apoptosis. Proc Natl Acad Sci USA. 2005;102:2028-33. (FC)
- 4. Sharabi A, Mozes E. The suppression of murine lupus by a tolerogenic peptide involves Foxp3-expressing CD8 cells that are required for the optimal induction and function of Foxp3-expressing CD4 cells. J Immunol. 2008;181:3243-51. (FC)
- 5. Laronne-Bar-On A, Zipori D, Haran-Ghera N. Increased regulatory versus effector T cell development is associated with thymus atrophy in mouse models of multiple myeloma. J Immunol. 2008;181:3714-24. (FC)
- 6. Barnes MJ, Krebs P, Harris N, Eidenschenk C, Gonzalez-Quintal R, Arnold CN, et al. Commitment to the regulatory T cell lineage requires CARMA1 in the thymus but not in the periphery. PloS Biol. 2009;7(3):e1000051. (FC)
- 7. Scalapino KJ, Daikh DI. Suppression of glomerulonephritis in NZB/NZW lupus prone mice by adoptive transfer of ex vivo expanded regulatory T cells. PLoS One. 2009;4(6):e6031. (FC)
- 8. Wafula PO, Teles A, Schumacher A, Pohl K, Yagita H, Volk H, et al. PD-1 but not CTLA-4 blockage abrogates the protective effect of regulatory T cells in a pregnancy murine model. Am J Reprod Immunol. 2009;62:283-92. (FC)
- 9. Wang D, Zhou R, Yao Y, Zhu X, Yin Y, Zhao G, et al. Stimulation of α7 nicotinic acetylcholine receptor by nicotine increases suppressive capacity of naturally occurring CD4+CD25+ regulatory T cells in mice in vitro. J Pharmacol Exp Ther. 2010;335:553-61. (FC)
- 10. Zhang Y, Yao Y, Huang L, Dong N, Yu Y, Sheng Z. The potential effect and mechanism of high-mobility group box 1 protein on regulatory T cell-mediated immunosuppression. J Interferon Cytokine Res. 2011;31:249-57. (FC)
- 11. Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res. 2013;73:6900-12. (FC)
See All References