Share a link
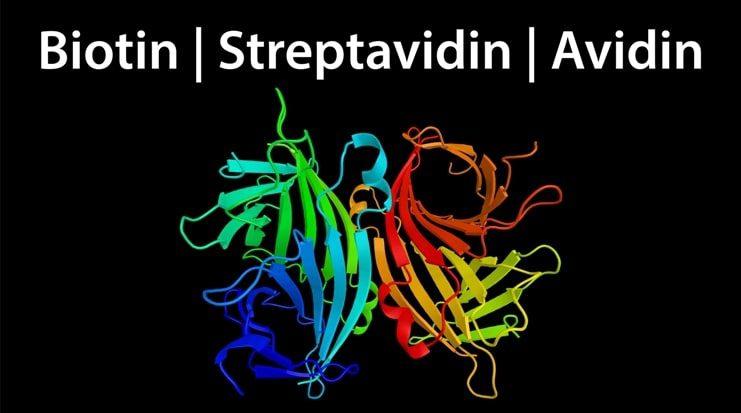
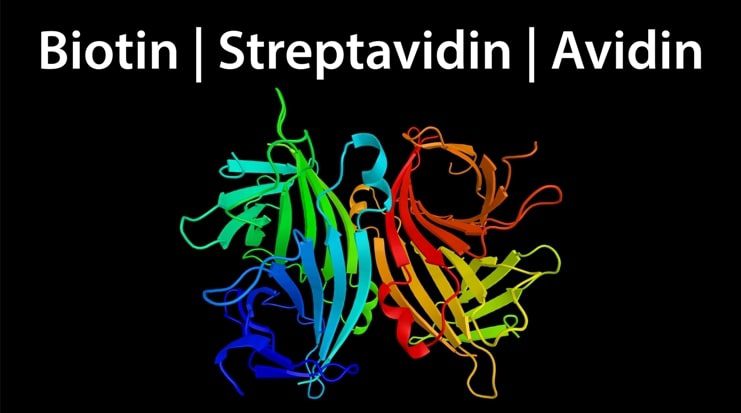
Streptavidin/Avidin-Biotin Interaction
The high affinity interaction between biotin and avidin/streptavidin has been widely utilized for scientific research for decades. It is used for applications including Western blot, ELISA, flow cytometry, and immunohistochemistry (IHC), as well as for protein isolation and enrichment.
What is biotin?
Biotin, also known as vitamin B7, is an essential nutrient found in foods such as eggs, liver, and cereal grains. It serves as a cofactor for the various carboxylase enzymes involved in metabolizing glucose, amino acids, and fats, and also regulates gene expression by modifying the activity of transcription factors. With a molecular weight of just 0.244 kDa, and a valeric acid side chain that is open to chemical modification, biotin can be conjugated to antibodies, proteins, and other biomolecules without altering their structure or function.
What is avidin?
Avidin is a protein found in the egg whites of birds, reptiles, and amphibians. It is comprised of four identical subunits, each with a molecular weight of approximately 16 kDa and the ability to bind a single biotin molecule. The avidin-biotin bond is the strongest known non-covalent interaction (Kd ~ 10-15 M), and can withstand denaturing conditions and extremes of temperature and pH. For this reason, it has long been harnessed for scientific research. However, a major limitation of avidin is that it can exhibit significant non-specific binding due to its basic isoelectric point (pI = 10.5) and high carbohydrate content. To address this problem, alternative forms of avidin have been developed, including Neutralite avidin, which is both neutral and deglycosylated.
What is streptavidin?
Streptavidin is a ~ 60 kDa biotin-binding protein which was originally isolated from the culture medium of Streptomyces avidinii but is now more often recombinantly produced. Like avidin, streptavidin is tetrameric and binds biotin with a comparably high affinity, yet the two proteins share just 33% sequence homology. Streptavidin is often preferred to native avidin from egg white for scientific research as it is non-glycosylated and has a near neutral isoelectric point, which preclude its interaction with carbohydrate-binding proteins (lectins) and negatively charged biomolecules. However, it is important to note that streptavidin contains a tripeptide sequence (RYD) which can cause unwanted background signal in some cytochemical and histochemical applications through binding to cell surface receptors.
What are some common applications for the biotin-avidin/streptavidin interaction?
One of the main research uses for the biotin-avidin/streptavidin interaction is to detect proteins via methods such as Western blot, ELISA, flow cytometry, and IHC. This typically involves using biotinylated primary or secondary antibodies for target detection, whereby the addition of a conjugate consisting of avidin/streptavidin bound to an enzyme or fluorescent dye produces a measurable signal. Because each antibody is labeled with multiple biotin molecules, and each avidin/streptavidin molecule can bind four biotins, an advantage of this method is that it provides signal amplification.
The biotin-avidin/streptavidin interaction is also used for protein isolation and enrichment. Often, the sample material is incubated with a biotinylated primary antibody, prior to the addition of avidin/streptavidin coated agarose beads and centrifugation to extract the target of interest from solution. Compared to methods which use Protein A or Protein G agarose beads, incubation times are shorter and target antigen recovery is not limited by endogenous antibodies binding to the bead surface.
What is the ABC staining method?
The avidin-biotin complex (ABC) staining method is a 3-step detection method used during IHC to boost assay sensitivity. First, the sample is incubated with an analyte-specific primary antibody, then a biotinylated secondary antibody is added. Towards the end of the secondary antibody incubation step, avidin is pre-incubated with a biotinylated enzyme – either horseradish peroxidase (HRP) or alkaline phosphatase (AP) – to form multiple large avidin-biotinylated enzyme complexes. This mixture is then applied to the sample, followed by the addition of a chromogenic substrate, giving rise to an elevated signal at each antigenic site due to the high concentration of enzyme that is present.
What is the LSAB method?
The labeled streptavidin-biotin (LSAB) staining method is similar to the ABC method, but uses enzyme-streptavidin conjugates (HRP-streptavidin or AP-streptavidin) in place of the avidin-biotinylated enzyme complexes. It is reported to have up to 10 times greater sensitivity than the ABC method since the smaller complexes are better able to penetrate the sample material.
We offer an extensive selection of biotinylated secondary and primary antibodies, as well as Neutralite avidin and streptavidin conjugates, all of which are validated for use in multiple applications including ELISA, flow cytometry, Western blot, IHC, and ELISpot.